Substrate and enzyme concentration
The normal pattern of dependence of enzyme rate on substrate concentration ([S]) is which at low substrate concentrations a doubling of [S] will lead to a doubling of the initial velocity (V0). Though, at higher substrate concentrations the enzyme becomes further and saturated increases in [S] lead to very small changes in V0. This happens because at saturating substrate concentrations effectively all of the enzyme molecules have bound substrate. The whole enzyme rate is now dependent on the rate at that the product can dissociate from the enzyme and adding additional substrate will not affect this. The shape of the consequential graph when V0 is plotted against [S] is known a hyperbolic curve.
In conditions where the substrate concentration is saturating for instance the entire enzyme molecules are bound to substrate a repetition of the enzyme concentration will lead to a doubling of V0. This will gives a straight line graph when V0 is plotted against enzyme concentration.
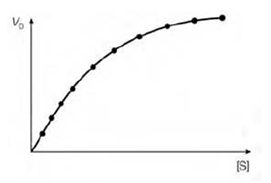
Figure: The relationship between substrate concentration [S] and initial reaction velocity (V0).
even little changes in the 3-D shape of the enzyme can alter the structure of the active site and lead to a reduce in catalytic activity. The whole effect of an increase in temperature on the reaction rate of the enzyme is a balance among these two opposing effects. The graph of temperature plotted against V0 will thus show a curve with a well-de?ned temperature optimum. For several mammalian enzymes this is around 37.C but there are also organisms that have enzymes adapted to working at considerably lower or higher temperatures. For instance, Taq polymerase which is used in the polymerase chain reaction, is found in a bacterium which lives at high temperatures in hot springs and therefore is adapted to work optimally at high temperatures.