Reversible competitive inhibition
A competitive inhibitor classically has nearly structural similarities to the common substrate for the enzyme. Therefore it competes with substrate molecules to bind to the active site. The enzyme may bind either an inhibitor molecule or a substrate molecule, but not both at the similar time. It binds reversibly to the active site. In high substrate concentrations the action of a competitive inhibitor is reduce because a sufficiently high substrate concentration will successfully compete out the inhibitor molecule in
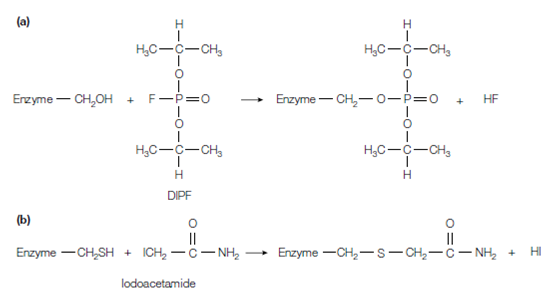
Figure: Structure and mechanism of action of (a) diisopropylphosphofiuoridate (DIPF) and (b) iodoacetamide.
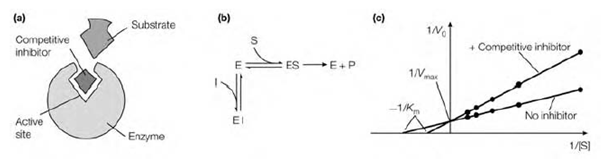
Figure: The characteristics of competitive inhibition. (a) A competitive inhibitor competes with the substrate for binding at the active site of the enzyme; (b) the enzyme can bind either substrate or the competitive inhibitor but not both; (c) Lineweaver-Burk plot showing the effect of a competitive inhibitor on Km and Vmax.
binding to the active site. Therefore there is no modification in the Vmax of the enzyme but the apparent affinity of the enzyme for its substrate reduce in the presence of the competitive inhibitor, and thus Km increases.
A well example of competitive inhibition is gives through succinate dehydrogenase. That enzyme uses succinate as its substrate and is competitively inhibited through malonate that differs from succinate in having one rather than two methylene groups that is described in above figure. Several drugs work through mimicking the structure of the substrate of a goal enzyme, and thus act as competitive inhibitors of the enzyme. The Competitive inhibition can be recognized through using a Lineweaver–Burk plot. V0 is measured at varient substrate concentrations in the presence of a fixed concentration of inhibitor. A competitive inhibitor rise the slope of the line on the Lineweaver–Burk plot and alters the intercept on the x-axis because Km is increased but leaves the intercept on the y-axis not changed (because Vmax remains constant).