The carbon cycle
The environmental cycling of carbon compounds includes a flux of above 2×1014 kg C per year, very much larger than for any other substance apart from water (approximately 5×1017 kg per year in the hydrological cycle). Compassionate the carbon cycle has become particularly urgent like the atmospheric CO2 content is presently increasing, producing global warming via the trapping of IR radiation within the atmosphere. Figure 1 depicts a summary of the major processes, along with estimates of the reservoirs (square boxes) and annual fluxes (round-cornered boxes) in units of 1012 kg C.
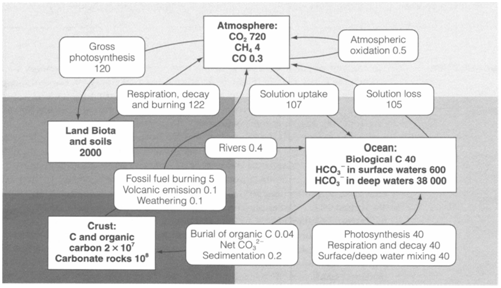
Fig. 1. The carbon cycle, showing reservoirs (square-cornered boxes) and annual fluxes (round-cornered boxes) in units of 1012 kg C.
Atmospheric CO2 is cycled in approximately equal amounts through two different processes: (i) the conversion into soluble bicarbonate HCO-3and the consequent regeneration of CO2 while water evaporates; (ii) the conversion into biological carbon compounds through photosynthesis, and reoxidation to CO2 through respiration. Anaerobic decay of vegetation, ruminant animals like cows and other natural processes make small amounts of CH4 and CO that are oxidized within the atmosphere to CO2. Several parts of the cycle operate along with much larger reservoirs of carbon, but also very much slowly: they involve the mixing of surface bicarbonate along with deep ocean waters, the creation of sedimentary carbonate rocks (mainly from CaCO3 shells and skeletons of marine organisms) and the eventual decomposition of carbonates through heating deep within the crust to again generate CO2.
Dissimilar parts of the natural cycle must be almost in balance, even though over a period of millions of years a number of organic carbon has been buried before reoxidation, providing fossil fuels consisting of reduced carbon in the crust. Dioxygen from photosynthesis have passed into the atmosphere, although over geological time most of it has been exhausted in oxidizing surface rocks (mainly FeII to FeIII compounds, and sulfides to sulfates), just a small fraction remaining like free O2. The burning of fossil fuels comprise opposed this natural tendency and currently transfers approximately 5×1012 kg C per year into the atmosphere like CO2. Parts of the cycle outside human control might be responding to start some of this additional input, although the ability of either surface ocean waters or life to hold it in the short term is extremely limited, and the burning of land vegetation gives to the problem through reducing photosynthesis. Even though excess CO2 must finally return to the crust like carbonate minerals, which can take place only over time scales measured in thousands or even millions of years.