Stabilization:
Because carbon is not electronegative, it cannot stabilize the charge. Though, stabilization is possible by resonance. The lone pair of electrons on the carbanion creates a new π bond to the carbonyl carbon. Since this bond is created, the weak π bond of the carbonyl group breaks and both these electrons move onto the oxygen. This results in the negative charge ending up on the electronegative oxygen in which it is more stable. This technique is exactly similar as the one described for the carboxylate ion. Though, whereas both resonance structures are evenly stable in the carboxylate ion, this is not the case here. The resonance structure comprising the charge on the oxygen atom (an enolate ion) is much more stable than the original carbanion resonance structure. Hence, the enolate ion will predominate over the carbanion.
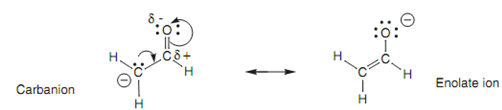
Figure: Resonance interaction between carbanion and enolate ion.