Loss of a proton and formation of a carbanion:
Treatment with a base results in loss of one of the acidic α proton (Fig.).
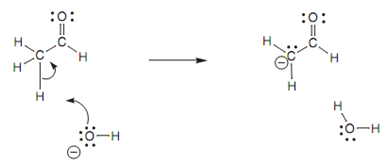
Figure: Loss of α proton and formation of a carbanion.
A lone pair on the hydroxide oxygen creates a new bond to α proton. At the similar time as this occurs, the C-H bond breaks. Both electrons of that bond end up on the carbon atom and provide it a lone pair of electrons and a negative charge (a carbanion). Though, carbanions are generally very reactive, unstable species that are not easily formed. Hence, some form of stabilization is included here.