Claisen condensation:
The Claisen reaction includes the condensation or linking of two ester molecules to make a β-ketoester as shown in diagram.
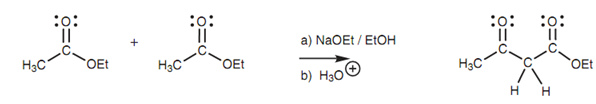
Figure: Claisen condensation.
This reaction can be observed like the ester equivalent of the Aldol reaction. The reaction includes the creation of an enolate ion from one ester molecule that then goes through nucleophilic substitution along with a second ester molecule.
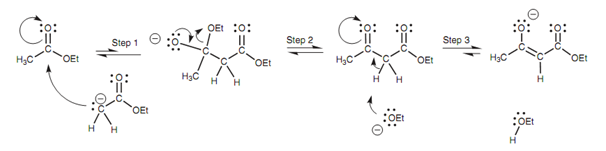
Figure: Mechanism of the Claisen condensation.
The ethoxide ion that is made in step 2 removes an α-proton from the β-ketoester in step 3 to make a stable enolate ion and this drives the reaction to completion. The last product is isolated from protonating the enolate ion with acid.