Alkylations:
Enolate ions may be alkylated with alkyl halides by the SN2 nucleophilic substitution of an alkyl halide.
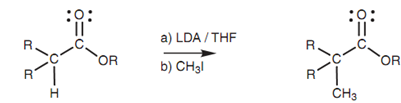
Figure: α-Alkylation of an ester.
Even though simple esters can be transformed to their enolate ions and alkylated, the make use of a molecule like diethyl malonate is far more effective. This is since the α-protons of diethyl malonate (pKa 10-12) are more acidic as compared to the α-protons of a simple ester like ethyl acetate (pKa 25) and can be removed via a milder base. It is feasible to predict the base needed to perform the deprotonation reaction by considering the pKa value of the conjugate acid for that base. If this pKa is higher as compared to the pKa value of the ester, after that the deprotonation reaction is possible. For instance, the conjugate acid of the ethoxide ion is ethanol (pKa 16) and thus any ester having a pKa less than 16 will be deprotonated through the ethoxide ion. Hence, diethyl malonate is deprotonated but not ethyl acetate.
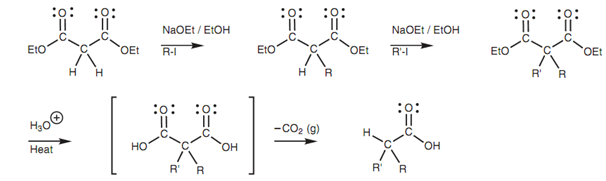
Figure: Alkylations of diethyl malonate.