Resonance structures for the conjugate base:
Though, the acidity of the α proton is increased if it is flanked by two carbonyl groups than one, for instance, 1,3-diketones (β-diketones) or 1,3- diesters (β-keto esters). This is since the negative charge of the enolate ion can be stabilized through both carbonyl groups resulting in three resonance structures. For instance, the pKa of 2,4-pentanedione is 9.
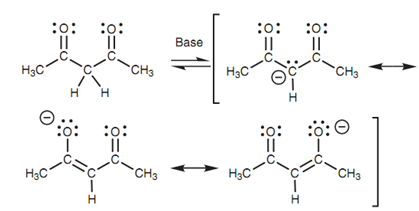
Figure: Resonance structures for the conjugate base of a 1,3-diketone.