Orbital interactions:
Though, in cyclic systems, the hydrogen atoms are locked in space and the relative stereochemistry is significant if α proton is to be acidic.
Enolate ions created from ketones or aldehydes are very important in the synthesis of more complex organic molecules. The effortlessness with which an enolate ion is created is related to the acidity of α proton. The pKa of propanone (acetone) is »19.3 that mean that it is a stronger acid compared to ethane (pKa »60) and a much weaker acid as compared with the acetic acid (pKa 4.7). the meaning of this is that strong bases like sodium hydride, sodium amide, and lithium diiso-propylamide (LiN(i-C3H7)2) are needed to create an enolate ion.
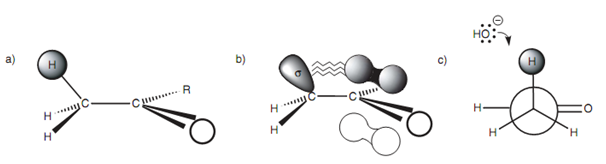
Figure: (a) α proton; (b) orbital diagram illustrating orbital interactions; (c) Newman projection.