Heat
The Heat, similar to work, is energy in transit. The transfer of energy as heat, though, takes place at the molecular level as an outcome of a temperature difference. The symbol Q is used to symbolize heat. In engineering applications, the standard unit of heat is the British thermal unit (Btu). Particularly, this is termed as the 60 degree Btu since it is measured by a one degree temperature alters from 59.5 to 60.5°F.
Since with work, the quantity of heat transferred based upon the path and not simply on the first and final conditions of the system. Also, since with work, it is significant to differentiate among heat added to a system from its surroundings and heat eliminated from a system to its surroundings. A positive value for heat points out that heat is added to the system by its surroundings. This is in disparity to work which is positive whenever energy is transferred from the system and negative whenever transferred to the system. The symbol q is at times used to point out the heat added to or eliminated from a system per unit mass. It equivalents the total heat (Q) added or eliminated divided by the mass (m). The word "specific heat" is not used for q as specific heat is used for other parameter. The quantity symbolized by q is termed to simply as the heat transferred per unit mass.
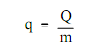
Here:
q = heat transferred per unit mass (Btu/lbm)
Q = heat transferred (Btu)
m = mass (lbm)
The finest way to enumerate the definition of heat is to consider the association among the quantity of heat added to or eliminated from a system and alter in the temperature of the system. Everyone is well-known with the physical phenomena which when a substance is heated, its temperature rises, and whenever it is cooled, its temperature reduces. The heat added to or eliminated from a substance to generate a change in its temperature is termed as sensible heat. The units of heat are frequently defined in terms of the changes in temperature it generates.
The other type of heat is termed as latent heat. The latent heat is the quantity of heat added to or eliminated from a substance to generate a change in phase. Whenever latent heat is added, no temperature change takes place. There are two kinds of latent heat. The primary is the latent heat of fusion. This is the quantity of heat added or eliminated to change phase among solid and liquid. The second kind of latent heat is the latent heat of vaporization. This is the quantity of heat added or eliminated to change phase among liquid and vapor. The latent heat of vaporization is at times termed as the latent heat of condensation.
Various substances are affected to diverse magnitudes by the addition of heat. Whenever a given quantity of heat is added to various substances, their temperatures rise by various amounts. The ratio of the heat (Q) added to or eliminated from a substance to the change in temperature (?T) generated is termed as the heat capacity (Cp) of the substance. The heat capacity of a material per unit mass is termed as the specific heat (cp) of the substance. The subscript p points out that the heat capacity and specific heat exert whenever the heat is added or eliminated at constant pressure.
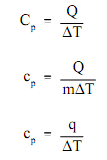
Here:
Cp = heat capacity at constant pressure (Btu/°F)
cp = specific heat at constant pressure (Btu/lbm-°F)
Q = heat transferred (Btu)
q = heat transferred per unit mass (Btu/lbm)
m = mass (lbm)
?T = temperature change (°F)
One lbm of water is increased 1°F and one Btu of heat is added. This entails that the specific heat (cp) of water is one Btu/lbm-°F. The cp of water is equivalent to one Btu/lbm-°F only at 39.1°F.
By rearranging the equation we acquire Q=mcp?T, that is used to compute latent heat. By replacing mass flow rate in lbm/hr, for m, we acquire. Q - mcp?T. This equation is used to compute heat transfer in Btu/hr.