Fraction of atoms in the excited states:
Table: Fraction of atoms in the excited states of different elements at various temperatures
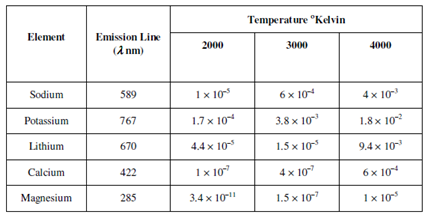
The number of atoms within the excited state increases extremely rapidly along with increase within temperature as displays in Table. Thus, flames along with higher temperatures will raise the number of excited atoms and make the method more sensitive. Higher temperatures are also needed for decomposition of the compound having high lattice energies within atoms. Therefore, higher temperatures might also cause ionisation of the atoms which might cause interference within the determination. We will take up that issue later in this unit. Thus, we required to optimise the temperature of the flame to be used.
We have so far learnt about the origin and categorization of atomic spectroscopic methods, the features of atomic spectrum and the principle of flame photometry. Since flame has a crucial role to play in flame photometry, now let us learn about the flame and its features before taking up the instrumentation needed for the measurement of atomic spectra.