Example
Compute the heat transferred per unit mass when 1500 Btu's are transferred to 40 lbm of water.
Solution:
By using the equation
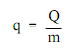
q - (1500Btu / 40 lbm)
q - 37.5 Btu/lbm
Example
Compute how much heat is needed to increase the temperature of 5 lbm of water from 50°F to 150°F? (Suppose the specific heat (cp) for water is constant at 1.0 Btu/lbm-°F.)
Solution:
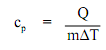
Q= cpm?T
Q = (1.0 Btu/lbm-°F)(5 lbm)(150°F - 50°F)
Q = (1.0 Btu/lbm-°F)(5 lbm)(100°F)
Q = 500 Btu