Entropy
The Entropy (S) is a property of a substance, since are temperature, pressure, volume, and enthalpy. Since entropy is a property, modifications in it can be established by knowing the first and final circumstances of a substance. Entropy measures the energy of a substance which is no longer available to perform helpful work. Since entropy tells too much about the helpfulness of a quantity of heat transferred in executing work, the steam tables involve values of specific entropy (s = S/m) as portion of the information tabulated. The entropy is at times referred to as a measure of the incapability to do work for a certain heat transferred. Entropy is symbolized by the letter S and can be defined as ?S in the relationships below.
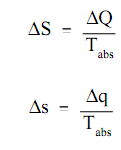
Here:
S = the alter in entropy of a system during some procedure (Btu/°R)
?Q = the quantity of heat transferred to or from the system during the procedure (Btu)
Tabs = the absolute temperature at which the heat was transferred (°R)
?s = the change in specific entropy of a system during some procedure (Btu/lbm -oR)
?q = the amount of heat transferred to or from the system during the process (Btu/lbm)
Similar to entropy, enthalpy cannot be measured directly. Also, similar to enthalpy, the entropy of a substance is provided with respect to some reference value. For illustration, the specific entropy of water or steam is given by using the reference which the specific entropy of water is zero at 32°F. The fact which the absolute value of specific entropy is unknown is not a problem since it is the change in specific entropy (?s) and not the absolute value which is significant in practical problems.