Concentration profiles at different stages of elution:
In this way, continued addition of solvent carries the solute molecules down the column in a continuous series of transfers between the mobile and stationary phases. The solute movement can only occur in the mobile phase, therefore, the average rate at which a solute zone migrates down the column depends upon fraction of time it spends in that phase. This fraction of time spent is small for solutes which are strongly retained by the stationary phase. If we refer to Figure, this is the situation for Y. On the other hand, the retention of X is more in the mobile phase. As a result of this difference in retention rates, a component of mixture separate within bands or zones, (time t2). If sufficient quantity of solvent is passed, the two species are isolated as individual zones and then pass out of the column where they can be detected or collected. (time t3 and time t4).
It should be noticed that during the separation procedure, the dilution of analyte takes place. The analytes get more dilute than they were present in the original mixture. Therefore, the detectors employed for the separated components should be invariably more sensitive than would be required if the separation was not needed. The detector responds to the concentration of the solute coming out of the column. When its signal is plotted against time or the volume of the mobile phase a series of peaks are plotted. Such a plot is known as a chromatogram. The chromatogram is useful for both qualitative and quantitative analysis. A position of the peak on time axis helps to recognize the elements and the areas under the peak provide the amount of each component.
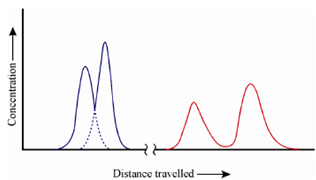
Figure: Concentration profiles at different stages of elution
In the separation of X and Y, the species Y is more strongly held by the column and thus it lags. During the movement in the column the distance between the two zones increases. At the same time, broadening of two zones takes place. This lowers the efficiency of the separation method.