Example of Zaitsev’s rule:
If the elimination takes place by the E1 mechanism, the reaction is much more likely to compete with the SN1 reaction and a mixture of substitution and elimination products is likely.
The E2 elimination needs the existence of a β-proton. If there are various options available, a mixture of alkenes will be acquired, but the favored alkene will be the most substituted (and most stable) one (Zaitsev's rule).
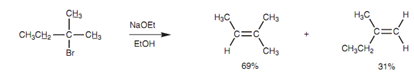
Figure: Example of Zaitsev's rule.
For the reaction the transition state resemble the product more than the reactant and thus the factors that stabilize the product as well stabilize the transition state and make that specific route more likely. Though, the opposite preference is found while potassium tert-butoxide is employed as base, and the less substituted alkene is favored.