Hofmann elimination of a secondary amine:
The cause for this preference is not completely understood, but may have something to do with the large bulk of the triethylamine leaving group hindering the approach of the hydroxide ion like that it approaches the least hindered β-carbon.
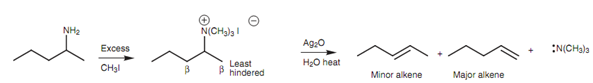
Figure: The less substituted alkene is preferred in the Hofmann elimination.
Secondary and tertiary amines can as well be exhaustively methylated then treated with silver oxide. Though, mixtures of dissimilar alkenes may be acquired if the N-substituents are different alkyl groups.
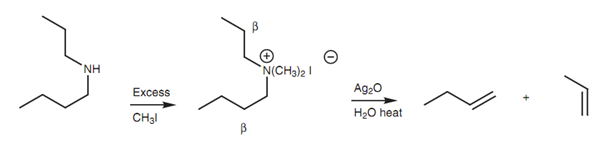
Figure: Hofmann elimination of a secondary amine.
The Hofmann elimination is not probable with primary arylamines, but secondary and tertiary arylamines will react if one of the substituents is an appropriate alkyl group. After that elimination of the aromatic amine can take place such that the alkyl substituent is converted to the alkene.
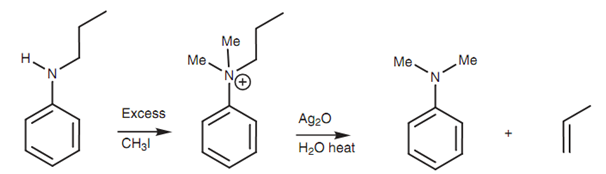
Figure: Hofmann elimination of an aromatic amine