Elimination of alcohols obeys Zaitsev’s rule:
Elimination under acidic conditions is much more successful since the hydroxyl group is ?rst protonated and after that departs the molecule like a neutral water molecule (dehydration) that is a much better leaving group. If different isomeric alkenes are probable, the most substituted alkene will be favored - another instance of Zaitsev's rule. The reaction acts best with tertiary alcohols because the elimination proceeds via the E1 mechanism.
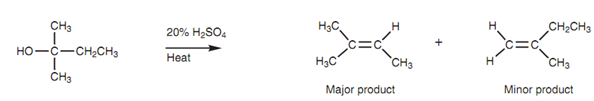
Figure: Elimination of alcohols obeys Zaitsev's rule.
The mechanism shown in figure involves the nucleophilic oxygen of the alcohol by using one of its lone pairs of electrons to make a bond to a proton to generate a charged intermediate (Step 1). Now that the oxygen is protonated, the molecule has a better leaving group as water can be ejected as a neutral molecule. The E1 mechanism can now carry on as normal. Water is lost and a carbocation is made (Step 2). After that water acts as a base in the second step, by using one of its lone pairs of electrons to make a bond to the β-proton of the carbocation.