E2 Mechanism:
The E2 mechanism is a concerted mechanism that including both the alkyl halide and the nucleophile. The result of it is that the reaction rate depends upon the concentration of both reagents and is described as second order (E2 Elimination second order). To demonstrate the mechanism, we shall have a look at the reaction of 2-bromopropane with a hydroxide ion as shown in figure.
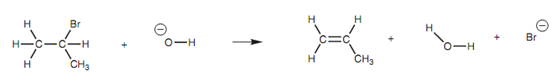
Figure: Reaction of 2-bromopropane with the hydroxide ion.
The mechanism shown in figure includes the hydroxide ion making a bond to the susceptible proton. Since the hydroxide ion forms its bond, the C-H bond breaks. Both of the electrons in that bond could move onto the carbon; however there is a neighboring electrophilic carbon that attracts the electrons and thus the electrons move in to make a π bond among the two carbons. At similar time as this π bond is made, the C-Br bond breaks and both electrons end up on the bromine atom that is lost as a bromide ion.
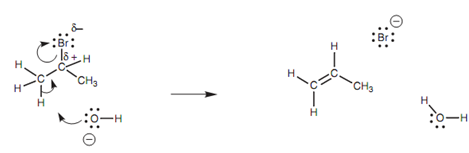
Figure: E2 Elimination mechanism.