E1 Elimination mechanism for alcohols:
The C-H bond is broken and both of the electrons in that bond are employed to make a π bond among the two carbons. Because this is an E1 reaction, tertiary alcohols react better than primary or secondary alcohols.
The E1 reaction is not perfect for the dehydration of primary or secondary alcohols as vigorous heating is needed to force the reaction and this can result in rearrangement reactions.
Hence, alternative methods are helpful. Reagents like phosphorus oxychloride (POCl3) dehydrate secondary and tertiary alcohols within mild basic conditions by using pyridine as solvent.
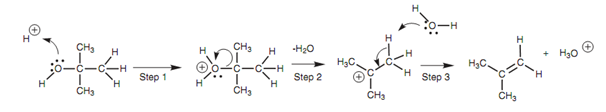
Figure: E1 Elimination mechanism for alcohols.
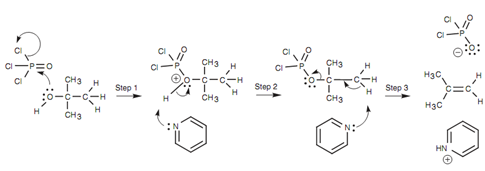
Figure: Mechanism for the POCl3 dehydration of an alcohol.
The phosphorus oxychloride works to activate the alcohol, transforming the hydroxyl function into a better leaving group. The mechanism includes the alcohol acting like a nucleophile in the first step. Oxygen employs a lone pair of electrons to make a bond to the electrophilic phosphorus of POCl3 and a chloride ion is lost (Step 1). After that Pyridine removes a proton from the structure to make a dichlorophosphate intermediate (Step 2). The dichlorophosphate group is a much better leaving group as compared to the hydroxide ion and thus a normal E2 reaction can occur. Pyridine works as a base to remove a β-proton and as this is happening, the electrons from the old C-H bond are employed to make a π bond and eject the leaving group (Step 3).