Tertiary alkyl:
Tertiary alkyl halides are necessarily not reactive to strong nucleophiles in polar, halides aprotic solvents - the conditions for the SN2 reaction. Tertiary alkyl halides can go through E2 reactions while treated with a strong base in a protic solvent and will do so in good yield because the SN2 reaction is very much highly disfavored. Within non basic conditions in a protic solvent, E1 elimination and SN1 substitution both occur.
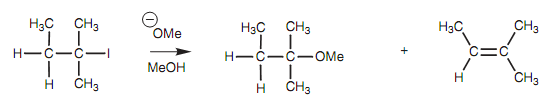
Figure: Reaction of 1-iodo-2-methylbutane with methoxide ion.
A tertiary alkyl halide treated along with sodium methoxide could provide ether or an alkene. A protic solvent is employed here and this favors both the SN1 and E1 mechanisms. Though, a strong base is as well being used and this favors the E2 mechanism. Hence, the alkene would be supposed to be the main product with only a very small amount of substitution product that arising from the SN2 reaction. Heating similar alkyl halide in methanol alone means that the reaction is being performed in a protic solvent with a non basic nucleophile (MeOH). These situations would result in a mixture of substitution and elimination products taking place from the SN1 and E1 mechanisms. The substitution product would be favored on the elimination product.