Primary alkyl halides:
Primary alkyl halides go through the SN2 reaction along with a large range of nucleophiles (e.g. RS-, I-, CN-, NH3, or Br-) in polar aprotic solvents like hexamethylphosphoramide (HMPA; [(CH3)2N]3PO). Though, there is all the time the possibility of some E2 elimination occurring also. However, substitution is generally favored over elimination, even while using strong bases such as HO- or EtO-. If E2 elimination of a primary halide is needed, it is best to make use of a strong bulky base like tert-butoxide [(CH3)3C-O-]. With a bulky base, the elimination product is favored on the substitution product because the bulky base experiences much more steric hindrance in its approach to the electrophilic carbon than it does to the acidic β-proton.
So, treatment of a primary halide with an ethoxide ion is likely to provide a mixture of an ether arising from SN2 substitution together with an alkene arising from E2 elimination, with the ether is being favored.
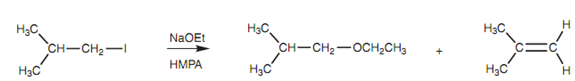
Figure: Reaction of 1-iodo-2-methylpropane with sodium ethoxide.
Through using sodium tert-butoxide instead, the preferences would be reversed.
Rising the temperature of the reaction shifts the balance from the SN2 reaction to the elimination reaction. This is since the elimination reaction has higher activation energy because of more bonds being broken. The SN1 and E1 reactions do not take place for primary alkyl halides.