Nonmetallic elements
As might be supposed from other features of its chemistry, the boron is exceptional and has elemental structures that cannot be understood in simple bonding terms. The simple concepts of electronpair stereochemistry and bonding explained in Topics C1 and C2 allow the structures to be rationalized although not always predicted, for the remaining non-metals. Single-bonded structures in which each element achieves an octet lead to the following predictions.
Group 14: four tetrahedral bonds as displayed in the diamond structure of Ge, C, Si, and Sn, and demonstrate in Fig..
Group 15: three bonds in a pyramidal (nonplanar) geometry, that can give rise to P4 molecules (white phosphorus) or a range of polymeric structures displayed by As and P. Phosphorus has various allotropes, some with actually complex structures but all are based on similar local bonding.
Group 16: two bonds, that are noncolinear, such as found in S8 rings and in spiral chains with Te and Se. The distinct allotropes of sulfur all have this bonding.
Group 17: one bond, providing diatomic molecular structures displayed by all the halogens.
Group 18: no bonds, leading to monatomic structures with atoms held only by van der Waals' forces. The general solid structure of the noble gas elements is fcc.
The structural chemistry of the period 2 elements C, N and O depicts a greater tendency to multiple bonding than in lower periods. Molecular N2 (triple bonded) and O2 (double bonded) are the general forms of these elements. With carbon, other allotropes additionally to diamond are feasible. The thermodynamically stable form at normal pressures is graphite (see Fig. 3b), in which some delocalized π bonding is exist along with the three a bonds formed by each atom. Fullerenes like C60 have similar bonding arrangements.
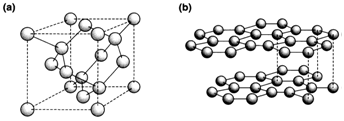
Fig. . Structures of (a) diamond and (b) graphite.
Other group trend with p-block elements is the increasing tendency in the direction of metallic character in lower periods. Like with the chemical trends, the alteration in structures and properties of the elements come out more of a continuous transition than a sharp borderline. The structural difference among near-neighbor (bonded) atoms and next-near-neighbor (nonbonded) ones becomes less marked down each group. Table 1 lists ratio of these distances for some nonmetallic elements of periods 3-5, and depicts how the two distances become almost equal for heavier elements, particularly with Te and Sb, that are close to the metallic borderline. The peculiar structures displayed by some p-block metals suggest that some affect of directional bonding persists in the metallic state.
Table 1. The ratio of next-near-neighbor to near-neighbor distances in some solid p-block elements
