SDS-PAGE
In SDS (sodium dodecyl sulfate)-PAGE, the proteins are coated and denatured with a whole negative charge [due to bound sodium dodecyl sulfate (SDS) molecules] and thus the basis for their separation is only their mass. The protein combination is first treated with a reducing agent like as dithiothreitol or2-mercaptoethanol to break all the disul?de bonds. The tough anionic detergent sodium dodecyl sulfate is then added that disrupts nearly all the noncovalent interactions in the protein, unfolding the polypeptide chain. Around one molecule of sodium dodecyl sulfate binds by its hydrophobic alkyl chain to the polypeptide
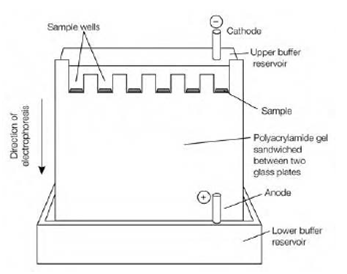
Figure: Polyacrylamide gel electrophoresis. The protein samples are loaded into the sample wells formed in the top of the gel. An electric field is applied across the gel from top to bottom and the proteins migrate down through the gel. The smaller the protein the further it will migrate.
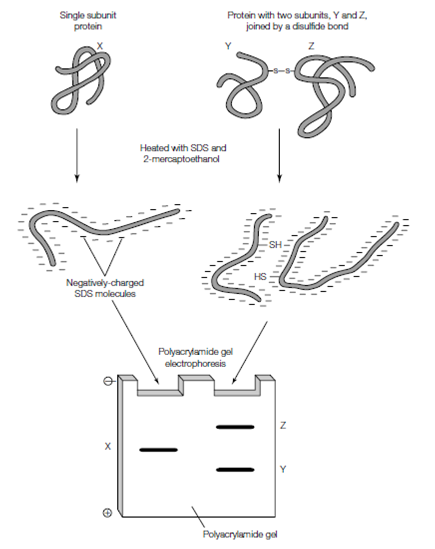
Figure: SDS-PAGE. The protein mixture is heated in the presence of 2-mercaptoethanol, which breaks any disul?de bonds, and SDS. The unfolded polypeptide chains are coated with the negatively-charged molecules of SDS and will migrate towards the anode on polyacrylamide gel electrophoresis. Smaller polypeptides migrate further through the gel than larger ones.
backbone for each two amino acid residues, that gives the denatured protein a large net negative charge which is proportional to its mass. The SDS or protein combination is then applied to sample wells in the top of a polyacrylamide gel in the figure. The buffer, that is the similar in both the lower and upper reservoirs and in the gel, has a pH of around 9, like in which the proteins have net negative charge and will migrate towards the anode in the lower reservoir. An electric current (around 300 V) is applied across the gel from top to bottom for 30–90 min in order to move the proteins by the gel in figure. After carrying out electrophoresis the gel is erased from the proteins and the apparatus described. Small proteins move furthest by the gel, while large ones move more slowly as they are held back through the cross-linking in the gel. Under these situations, the mobility of most polypeptide chains is linearly proportional to the logarithm of their mass. Therefore, if proteins of known molecular mass are electrophoresed alongside the templates the mass of the unknown proteins can be determined as there is a linear relationship among log10 of molecular distance and mass migrated by the gel. Proteins which differ in mass through about 2 percent (for instance 40 and 41 kDa; a difference of around 10 amino acid residues) can be illustrious under appropriate conditions. SDS- PAGE is a sensitive, rapid and broadly-used method that can be used to determine the degree of purity of a protein sample to estimate the molecular mass of an unknown protein and to deduce the number of polypeptide subunits within a protein.