Deactivating groups - resonance o/p directing:
The fourth and final group of aromatic substituents is the halogen substituents that deactivate the aromatic ring and that direct substitution to the ortho and para positions. These are maybe the trickiest to understand because they deactivate the ring through one effect, but direct substitution through a different effect. The halogen atom is powerfully electronegative and hence we would suppose it to have a strong electron-withdrawing inductive effect on the aromatic ring. This would create the aromatic ring less nucleophilic and less reactive to electrophiles. It would as well destabilize the needed intermediate for electrophilic substitution. Halogens are as well poorer nucleophiles and thus any resonance effects they might have are less significant than their inductive effects.
Though, if halogen atoms are deactivating the ring due to inductive effects, why do they not direct substitution to the Meta position such as other electron- withdrawing groups? Let us look at a particular reaction - the nitration of bromo- benzene.
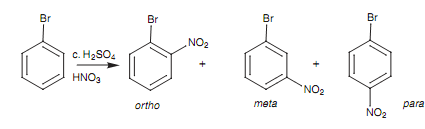
Figure: Nitration of bromobenzene.
There are 3 resonance structures for each of the three intermediates directing to these products, but the crucial ones to consider are those that position a positive charge next to the substituent. These take place with ortho and para substitution, but not meta substitution.