Activating groups - resonance o/p directing:
Phenol is an instance of a substituent that activates the aromatic ring through resonance effects and that directs substitution to the ortho and para positions. An electronegative oxygen atom is next to the aromatic ring, in phenol. Because oxygen is electronegative, it should comprise an electron-withdrawing inductive effect and thus might be supposed to deactivate the ring. The reality that the phenolic group is a powerful activating group is because of the fact that oxygen is electron rich and can as well act as a nucleophile, feeding electrons into the ring by a resonance process. As an instance, we shall look at the nitration of phenol.
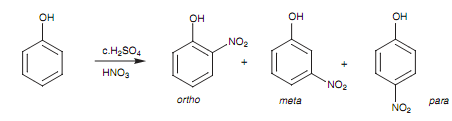
Figure: Nitration of phenol.
There are 3 resonance structures for the intermediate created in each type of electrophilic substitution, however there are two crucial ones to consider, occurring from ortho and para substitution. These resonance structures encompass the positive charge next to the OH substituent.
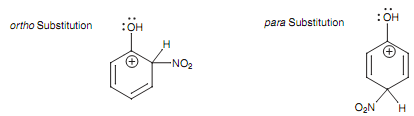
Figure: Resonance structures for the intermediates arising from ortho and para substitution.
If oxygen just only had an inductive effect, these resonance structures would be extremely unstable. Though, oxygen can work as a nucleophile and can make use of one of its lone pairs of electrons to create a new π bond to the neighboring electrophilic center. This results in a fourth resonance structure in which the positive charge is moved out of the ring and onto the oxygen atom. Delocalizing the charge such as this further stabilizes it and makes the reaction proceed more simply.