Activating groups - inductive o/p directing:
A methyl substituent is an instance of an inductive activating group and thus we shall consider once again the bromination of toluene. To describe the directing properties of the methyl group, we requires to look more closely at the mechanisms involved in producing the ortho, Meta, and para isomers. The preferred reaction pathway will be the one which undergoes the most stable intermediate. Because a methyl group directs ortho and para, the intermediates included in these reaction pathways are more stable as compared to the intermediate involved in Meta substitution. The subsequent intermediates and their resonance structures are displayed in diagram.
If we compare all the resonance structures above, we can mark one ortho and one para resonance structure (boxed) in which the positive charge is positioned directly next to the methyl substituent.
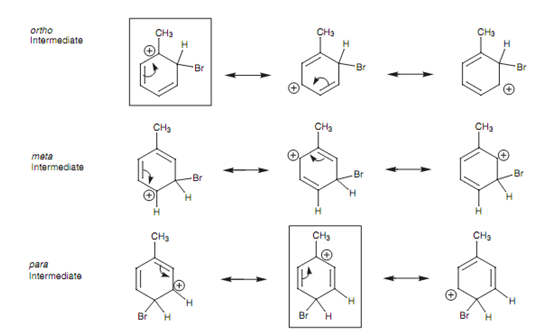
Figure: Intermediates for ortho, meta, and para substitution.