Mechanism:
In the mechanism the aromatic ring works as a nucleophile and gives two of its π electrons to create a bond to Br+. The aromatic ring has now lost one of its formal double bonds resultant in a positively charged carbon atom.
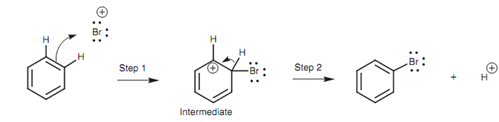
Figure: Mechanism of electrophilic substitution.
This 1st step in the mechanism is the same as the one explained for the electrophilic addition to alkenes, and thus the positively charged intermediate here is equal to the carbocation intermediate in electrophilic addition. Though, in step 2 the mechanisms of electrophilic addition and electrophilic substitution are different. While the carbocation intermediate from an alkene reacts along with a nucleophile to provide an addition product, the intermediate from the aromatic ring loses a proton. The C-H σ bond breaks and the two electrons move into the ring to again form the π bond, so regenerating the aromatic ring and neutralizing the positive charge on the carbon. This is the mechanism gone through in all electrophilic substitutions. The only variation is the nature of the electrophile.