Intermediate stabilization:
The rate-determining step in electrophilic substitution is the creation of the positively charged intermediate, and thus the rate of the reaction is ascertained through the energy level of the transition state leading to that intermediate. The transition state resembles the intermediate in character and thus any factor stabilizing the intermediate as well stabilizes the transition state and lowers the activation energy needed for the reaction. Hence, electrophilic substitution is more probable to occur if the positively charged intermediate can be stabilized. Stabilization is probable if the positive charge can be spread amongst dissimilar atoms - a process termed as delocalization. The process through which this can occur is termed as resonance.
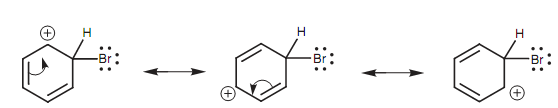
Figure: Resonance stabilization of the charged intermediate.
The resonance process includes two π electrons shifting their position round the ring to give the 'top' carbon with a fourth bond and so neutralize its positive charge. In the process, other carbon in the ring is left short of bonds and gains the positive charge. This procedure can be repeated like that the positive charge is spread to a third carbon. The structures drawn in figure are termed as resonance structures.