Halogenation:
The meaning of stable aromatic ring is that aromatic compounds are less reactive than alkenes to electrophiles. For instance, an alkene will react with Br2 while an aromatic ring will not. Hence, we have to activate the aromatic ring (that is make it a better nucleophile) or activate the Br2 (that is make it a better electrophile) if we want a reaction to take place. We will describe how electron-donating substituents on an aromatic ring raise the nucleophilicity of the aromatic ring. We shall observe how a Br2 molecule can be activated to make it a better electrophile. This can be completed through adding a Lewis acid like FeCl3, FeBr3, or AlCl3 to the reaction medium. These compounds all consist of a central atom (iron or aluminum) that is strongly electrophilic and does not have a full valence shell of electrons. The result of it is, the central atom can accept a lone pair of electrons, even from a weakly nucleophilic atom like a halogen. In the instance displayed in figure bromine uses a lone pair of electrons to create a bond to the Fe atom in FeBr3 and turns into positively charged. Bromine is now activated to behave like an electrophile and will react more simply with a nucleophile (the aromatic ring) through the normal mechanism for electrophilic substitution.
An aromatic ring can be chlorinated in an identical fashion, by using Cl2 in the existence of FeCl3.
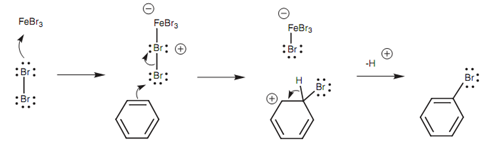
Figure: Mechanism by which a Lewis acid activates bromine towards electrophilic substitution.