Additions to terminal alkynes:
If a terminal alkyne is considered with an excess of hydrogen halide the halogens both end up on the more substituted carbon.
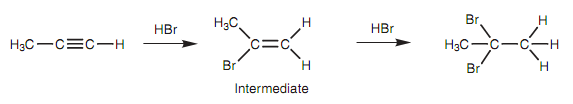
Figure: Reaction of propyne with HBr.
This is other instnace of Markovnikov's rule which says that the additional hydrogens end up on the carbon that already has the most hydrogen. Similar rule applies for the reaction with acid and mercuric sulfate that means that a ketone is created after keto-enol tautomerism instead of an aldehyde.
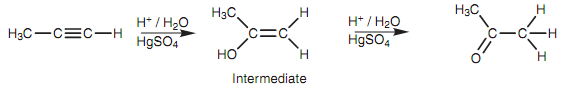
Figure: Reaction of propyne with aqueous acid and mercuric sulfate.