Mechanism:
The mechanism of 1, 4-addition starts off in similar way like a general electrophilic addition. We shall refer the reaction of a conjugated diene with hydrogen bromide as an instance. One of the alkene units of the diene employs its π electrons to create a bond to the electrophilic hydrogen of HBr.
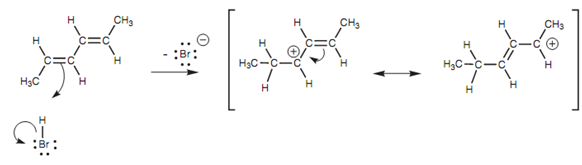
Figure: Mechanism of 1, 4-addition - first step.
The H-Br bond breaks at similar time to generate a bromide ion. The intermediate carbocation produced has a double bond next to the carbocation center and is termed as an allylic carbocation.
This type of system is now set up for resonance including the remaining alkene and the carbocation center, resultant in delocalization of the positive charge among the positions 2 and 4. Because of this delocalization, the carbocation is stabilized and this in turn describes two features of this reaction. Very first, the creation of two different products is now possible because the second stage of the mechanism includes the bromide anion attacking either at position 2 or at position 4.
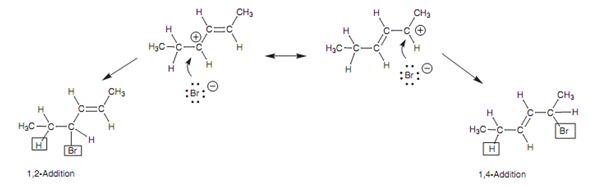
Figure: Mechanism of 1, 2- and 1,4-addition - second step.
Secondly, it describes why the alternative 1, 2-addition product is not created as displayed in diagram. The intermediate carbocation needed for this 1, 2-addition cannot be stabilized by resonance. Hence, the reaction proceeds via the allylic carbocation instead.
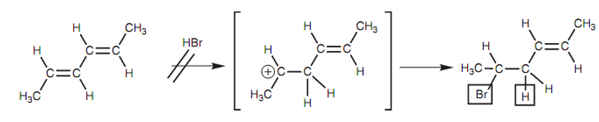
Figure: Unfavored reaction mechanism.