Carbocation Stabilities:
This reaction can be rationalized through proposing that the carbocation intermediate leading to product II is much more stable as compared to the carbocation intermediate leading to product I.
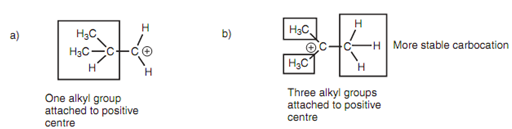
Figure: (a) Carbocation leading to product I; (b) carbocation leading to product II.
It is feasible to predict the more stable carbocation through counting the number of alkyl groups that attached to the positive center. The much more stable carbocation on the right has three alkyl substituents that are attached to the positively charged carbon while the less stable carbocation on the left only has one like alkyl substituent. The causes for this variation in stability are described in carbocation Stabilization, but the result is summarized through Markovnikov's rule. However, Markovnikov's rule does not all the time hold true. For instance, the reaction of CF3CH=CH2 with HBr provides CF3CH2CH2Br rather than CF3CHBrCH3. Here, the existence of electron-withdrawing fluorine substituents has a destabilizing effect on the two possible intermediate carbocations. The destabilizing effect will be much greater for the more substituted carbocation because the carbocation is closer to the fluorine substituents and thus the favoured carbocation is the least substituted one in this case.

Figure: Comparison of carbocations.