Reaction of HBr:
It has an electron-rich double bond consisting of four electrons, two of which build up a strong σ bond and two of which build up a weaker π bond. The double bond can be showed like a nucleophilic center. Hydrogen bromide comprises a polar H-Br bond and thus the hydrogen is an electrophilic center and the bromine is a nucleophilic center. Though, halogen atoms are very weak nucleophilic centers and thus this molecule is much more likely to react like an electrophile by its electrophilic hydrogen.
In the 1st step of electrophilic addition, the alkene works as a nucleophile and employs its two π electrons to create a new bond to the hydrogen of HBr. Since this new bond is created, the H-Br bond breaks because hydrogen is just only permitted one bond. Both electrons in that bond end up on the bromine atom to generate a bromide ion. Because the electrons from the π bond have been employed for the creation of a new σ bond, the π bond is no longer present. The result of it is that the 'left hand' carbon has been left with only three bonds and becomes positively charged. This is termed as a carbocation because the positive charge is on a carbon atom.
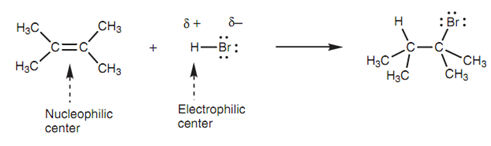
Figure: Reaction of HBr with 2, 3-dimethyl-2-butene.