Formation of the bromonium ion:
There is much more to this mechanism than meets the eye. The carbocation intermediate can be stabilized through neighboring alkyl groups by inductive and hyperconjugation effects. Though, it can as well be stabilized by sharing the positive charge with the bromine atom and a second carbon atom as shown in figure.
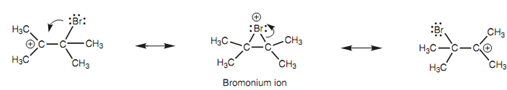
Figure: Formation of the bromonium ion.
The carbon that is positively charged is an electrophilic center. The bromine is a weak nucleophilic center. A neutral halogen does not generally work as a nucleophile, but in this case the halogen is held close to the carbocation creating reaction more likely. One time the lone pair of electrons on bromine is employed to create a bond to the carbocation, a bromonium ion is created in which the bromine gains a positive charge. The mechanism can go in opposite to again generate the original carbocation. Otherwise, the other carbon-bromine bond can break along with both electrons moving onto the bromine. This provides a second carbocation in which the other carbon bears the positive charge. So, the positive charge is shared among three different atoms and is further stabilized.