Anti-stereochemistry of bromine addition:
Proof for the presence of the bromonium ion is provided from the observation that bromine adds to cyclic alkenes (for example cyclopentene) in an anti-stereochemistry shown in figure.
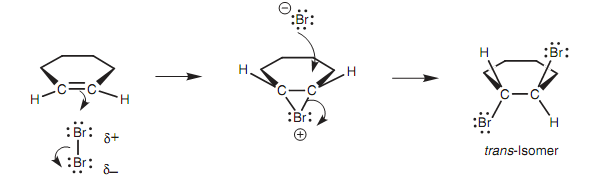
Figure: Anti-stereochemistry of bromine addition to a cyclic alkene.
Other words, every bromine adds to opposite faces of the alkene to generate only the trans isomer. None of the cis isomer is created. If the intermediate was a carbocation, a mixture of cis and trans isomers would be supposed because the second bromine could add from either side. Along with a bromonium ion, the second bromine must approach from the opposite side.