Alternative mechanism for electrophilic addition:
With a symmetrical alkene, the product is similar and thus it does not matter that end of the double bond is employed for the new bond to hydrogen. The chances are equivalent of the hydrogen adding to one side or another.
The electrophilic additions of H-Cl and H-I follow similar mechanism to generate alkyl chlorides and alkyl iodides correspondingly.
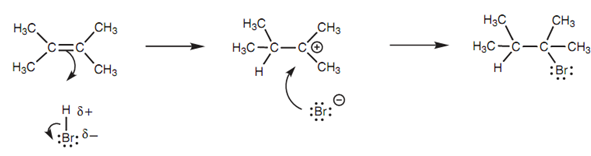
Figure: 'Alternative' mechanism for electrophilic addition of HBr to 2,3-dimethyl-2-butene.