Alkenes to alcohols:
Alkenes can be transformed to alcohols via treatment with aqueous acid (sulfuric or phosphoric acid). This electrophilic addition reaction includes the addition of water across the double bond. The hydrogen adds to one carbon when a hydroxyl group adds to another carbon.
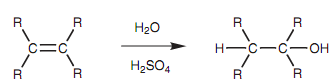
Figure: Synthesis of an alcohol from an alkene.
Occasionally the reaction conditions employed in this reaction are too harsh because heating is involved and rearrangement reactions can occur. A milder method that provides better results is to treat the alkene along with mercuric acetate [Hg(OAc)2] then sodium borohydride as displayed in figure.
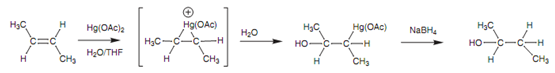
Figure: Synthesis of an alcohol from an alkene using mercuric acetate.
The reaction includes electrophilic addition of the mercury reagent to create an intermediate mercuronium ion. This reacts along with water to provide an organomercury intermediate. Reduction with sodium borohydride changes the mercury substituent with hydrogen and provides the final product.
Alkenes can as well be converted to alcohols through hydroboration.