Molecular orbitals for a conjugated diene:
The very easy and least energetic electronic transition is the promotion of one of the electrons from the π MO to the π* MO. The energy separation among the π MO and the π* MO is less as compared to that between an σ MO and an σ* MO, but for a simple alkene this corresponds to a wavelength of less than 200 nm that is still very much energetic for the vis-uv spectrum.
While it comes to conjugated systems, the energy variation among the HUMO and LUMO is very much reduced allowing electronic transitions to take place in the vis-uv region. Earlier we saw that it was feasible for the two π-orbitals in conjugated dienes to interact with each other. This interaction can effect in modified molecular orbitals as displayed in figure. The two π orbitals of the isolated alkenes interact to provide two new π orbitals π1 and π2 for the diene. An identical process occurs with the antibonding orbitals with the result that the energy variation among the HOMO and LUMO is reduced. For instance, the λmax for ethene is 171 nm while it is 217 nm for 1, 3-butadiene. (keep in mind that a higher wavelength means a lower energy.)
Since the amount of conjugation increases, the energy difference among the HOMO and the LUMO decreases until the difference is sufficiently small for transitions to take place in the visible region - resulting in a colored compound.
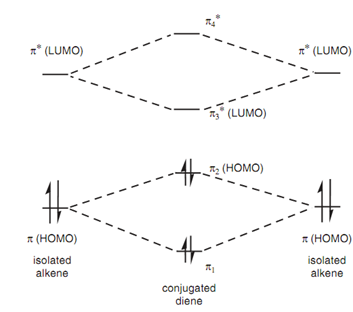
Figure: Molecular orbitals for a conjugated diene.