Carotene:
For instance, β-carotene is the precursor for Vitamin A and is accountable for the orange color in carrots. It has an absorption maximum of 497 nm that corresponds to blue-green. because this is the light that is absorbed, the color observed is what remains (that is red-orange).
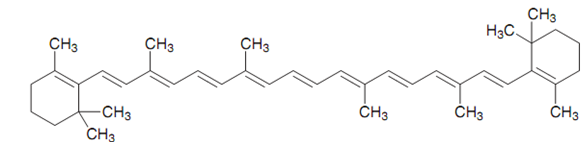
Figure: b-Carotene.
Along with conjugated dienes, the electronic transitions are because of π-π* transitions. If a heteroatom like oxygen is present (for example an unsaturated aldehyde), π-π* tran- sitions are still possible, but it is as well possible for one of the non-bonding electrons (that is from a lone pair of electrons) to be promoted to the LUMO. This transition is termed as n-π*. This transition includes less energy than a π-π* transition but the absorption bands observed in the spectrum are generally weaker in intensity than those because of π-π* transitions.