Resonance structures for an acid anhydride:
Even though the resonance effect is weak in esters and acid anhydrides, it can describe why acid anhydrides are much more reactive than esters. Acid anhydrides comprise two carbonyl groups and thus resonance can occur with either carbonyl group. The result of it is that the lone pair of the central oxygen is 'split' among both groups which mean that the resonance effect is split among both carbonyl groups. The meaning of this is that the effect of resonance at any one carbonyl group is diminished and it will keep strongly electrophilic. With an ester, there is just only one carbonyl group and thus it experiences the full impact of the resonance effect. Hence, its electrophilic strength will be diminished relative to an acid anhydride.
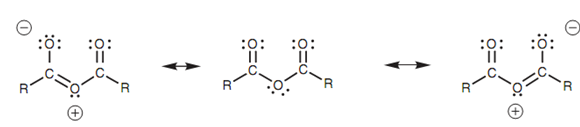
Figure: Resonance structures for an acid anhydride.