Steric factors:
Steric factors as well have a role to play in the reactivity of aldehydes and ketones. There are two methods of looking at this. One method is to look at the comparative ease with which the attacking nucleophile can move toward the carbonyl carbon. Another is to consider how steric factors affect the stability of the transition state leading to the last product.
Very first we consider the relative ease through which a nucleophile can approach the carbonyl carbon of an aldehyde and a ketone. To do that, we have to consider the bonding and the shape of these functional groups.
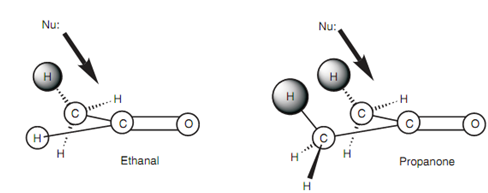
Figure: Steric factors.
Both molecules comprise a planar carbonyl group. The atoms that are in the plane are circled in white. A nucleophile will reach at the carbonyl group from above or below the plane. The figure below depicts a nucleophile attacking from above.
Note: on the neighboring methyl groups the hydrogen atoms are not in the plane of the carbonyl group and thus these atoms can hinder the approach of a nucleophile and so hinder the reaction.