Electronic factors:
Propanal encompass only one alkyl group feeding electrons into the carbonyl carbon, while propanone has two. Hence, the carbonyl carbon in propanal is much more electrophilic as compared to the carbonyl carbon in propanone.
As much more electrophilic the carbon, the much more reactive it is to nucleophiles. Hence, propanal is more reactive than propanone.
Electron inductive effects can be employed to describe differing reactivities among the different aldehydes. For instance the ?uorinated aldehyde is more reactive than ethanal. The fluorine atoms are electronegative and encompass an electron- withdrawing effect on the neighboring carbon, and creating it electron deficient. In turn this has an inductive effect on the neighboring carbonyl carbon. Because electrons are being withdrawn, the electrophilicity of the carbonyl carbon is raised, creating it more reactive to nucleophiles.
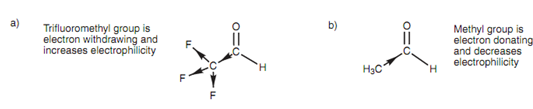
Figure: Inductive effect of (a) trifluoroethanal; (b) ethanal.