Definitions
Electronegativity may be explained as the power of an atom to attract electrons to itself in a chemical bond.
It is the most significant chemical parameter in determining the category of chemical bonds formed among atoms. It is difficult to quantify in a satisfactory way, particularly as electronegativity is not strictly a property of atoms on their own, but it depends to some extent on their state of chemical combination. Nevertheless various scales have been devised.
- Pauling electronegativity is based on bond energies, by the empirical observation that bonds among atoms with a large electronegativity variation tend to be stronger than those where the variation is small.
This scale was traditionally the first to be devised and even though it lacks a firm theoretical justification is still extensively used.
- Mulliken electronegativity is the average of the first ionization energy and the electron affinity of an atom, reflecting the significance of two possibilities in bond formation, losing an electron or gaining the other one. The scale has the benefit that electronegativity values can be estimated not only for the ground states of atoms, but for another electron configuration and even for polyatomic fragments.
- Allred-Rochow electronegativity is proportional to Zeff/r2, in which Zeff is the effective nuclear charge of valence orbitals, and r is the covalent radius of the atom. The value is proportional to effective electrostatic attraction on valence electrons by the nucleus, screened by the inner shell electrons.
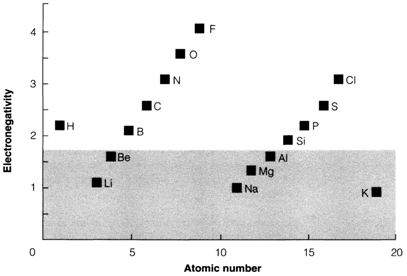
Fig. 1. Pauling electronegativity values for the elements H-K. Elements in the shaded region in the diagram are metallic..
Each scale produces distint numbers and they should not be mixed. The broad usual trends do, though, agree:
Electronegativity increases in the direction of the right and decreases in the direction of the bottom in the periodic table. So it follows similar trend as atomic ionization energies. Electropositive are the elements in early groups have low values. Diagram 1 depicts the Pauling electronegativities of elements up to potassium. Elements of the group 18 in early periods do not create any stable compounds and that's why the most electronegative element is fluorine.