The bonding triangle
The bonding triangle is a useful method of depicting how the electronegativities of two elements A and B (which may be similar) determine the kind of bond formed between them. The horizontal and vertical scales depict the Pauling electronegativities of the two elements. (Other scales would do uniformly well at this qualitative level.) Pure elements (A=B) show on the diagonal, and several compounds are displayed in the triangle. Three basic areas are distinguished.
- When A and B are both electropositive they create a metallic solid characterized by high electrical conductivity and a structure in which each atom is surrounded by sevearl others (often 12;). Metallic bonding involves the delocalization of electrons during the solid. The electrons are shared among atoms as in covalent bonding, but in a less particular way and without the directional character of covalent bonds.
- When A and B are both electronegative they create covalent compounds. These may contain individual molecules (O2, H2O, etc.) or of giant covalent lattices (polymeric solids) with a constant network of bonds. Even though the dividing line between these types is not sharp, very highly electronegative atoms (F, O, Cl, etc.) have more tendencies to molecular behaviour in both their elements and their compounds. Covalent solids do not carry out electricity well. The most significant characteristic of this bonding, whether in the molecules and solids, is its highly directional and particular nature. So the neighbours to any atom are limited in number (example four in the case of elemental silicon, 3 for phosphorus, 2 for sulfur, one for chlorine), and are usually found in specific.
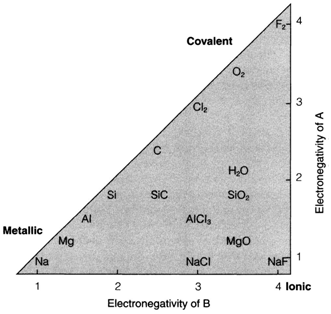
Fig. The bonding triangle, showing a selection of elements and compounds plotted against the Pauling electronegativities.
Geometrical arrangements. The simple most view of covalent bonding involves the sharing of electrons in particular localized bonds among atoms.
- When one atom is extremely electropositive and the other is very electronegative, a solid compound is formed that is frequently regarded as ionic. In this diagram there is a complete transfer of one or more electrons, giving cations of the electropositive anions and element of the electronegative one that are then held together by electrostatic attraction. Solids are created rather than molecules since the force is not directional and greatest stability is got by packing various anions around each cation and vice versa.