Resonance
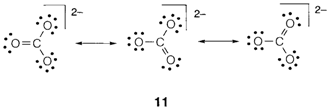
Sometimes there are more than one valence structure is possible and there appears to be no special assignment. A well known organic instance is in the disposition of double and single C-C bonds in benzene. In the carbonate ion CO32-) the three structures displayed are equal by symmetry and experimentally all three C-O bonds have equivalent length. We explain this condition as resonance between the dissimilar structures, and depict it by the double headed arrows displayed in 11. The term is misleading as it suggests a quick oscillation among different structures, which specifically does not occur. It is good to think of a wave function that is formed by combining the structures, none of which on their own explain the bonding correctly.
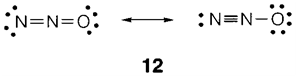
Resonance may also be suitable with different valence structures that are not equal but look equally plausible, like in nitrous oxide (N2O 12).
Atoms are frequently found in bonding situations that do not correspond to their 'normal' valency. Such type of cases can be rationalized by the concept of formal charge. A formal charge on an atom is necessarily the charge that would remain if all covalent bonds were broken with the electrons being assigned equally to the atoms involved. More mathematically, it is described as formal charge= (no. of the valence electrons in neutral atom)
-(no. of the nonbonding electrons)
-(1/2) (no. of the electrons in bonds formed)
The formal charges in CO and in the two valence structures for N2O are displayed in 13 and 14. The isoelectronic principle permits us to know these structures by analogy. So C- and O+ are both isoelectronic to neutral N and can identically create three bonds. The N2O structures can be understood with the isoelectronic relations N- and O (two bonds supposed), N+ and C (four bonds) and O- and F (one bond).
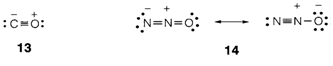
Formal charges are often drawn in organic structures; for instance, 'trivalent' carbon can take place as a carbocation (C+ isoelectronic to B, and with an incomplete octet) or a carbanion (C- isoelectronic to N, with a nonbonding pair).
They are not all the time written on inorganic valence structures, but the idea is helpful in judging the viability of a proposed structure. Some general principles are as follows:
- structures with no formal charges are preferred if possible;
- structures with the formal charges outside the range -1 to +1 are usually unfavorable;
- Negative formal charges should desirably be assigned to more electronegative atoms, positive charges to more electropositive atoms.
So in N2O (14), the structure with O- is probably more important than that with N-. The BF molecule (15) is isoelectronic with CO but the subsequent triple-bonded structure appears extremely unlikely because it needs formal charges B2- and F2+. The single-bonded create without charges may best explain the bonding.
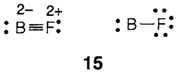
Formal charge is very dissimilar from oxidation state that is assigned by apportioning electrons in a bond to the more electronegative atom rather than similarly. Both are not natural assignments, helpful in their respective ways but neither is intended as a realistic judgment of the charges on atoms.