Octets and 'hypervalence'
A great majority of simple molecules consisting of the elements C-F of the second period can be depicted by Lewis structures with eight electrons around each of these atoms that are including all lone-pairs and shared electrons. The octet rule offers a systematization of the normal valencies of these elements: for an instance, a nitrogen atom has five electrons in its valence shell and so must share three more to achieve an octet, so forming three bonds. Hydrogen is limited to two electrons in its valence shell and these variations might be understood from the valence atomic orbitals available for electrons, the 1s only for H, 2s and 2p in the second period; the exclusion principle then limits the number of electrons that can be accommodated.

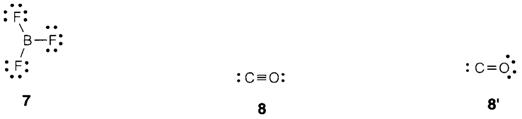
Several molecules containing boron (example BF3 7) have an incomplete octet and this has implications for their chemical reactivity. So, usually the structures with complete octets are preferred. So the triple-bonded representation for carbon monoxide (8) is better than the double-bonded one (8′) where carbon only has six valence-shell electrons.
Of the third and subsequent periods the nonmetallic elements create some compounds completely analogous to those of similar group in period 2. So we have H2S, H2Se and H2Te identical to H2O, all with octets. So, These heavier elements are able of octet expansion or hypervalence, the later term implying a valency higher than 'normal'. Instances are SF4 and SF6 (9, 10) where sulfur has correspondingly 10 and 12 electrons in its valence shell. Hypervalence is sometimes referred to be a consequence of the availability of further orbitals for bonding (example 3d in addition to 3s and 3p for sulfur). Even though this may play a part, it is usually thought that other variations between the periods are equally significant; particularly size and electronegativity.
