Lewis and valence structures
A single covalent bond is created when two atoms share a pair of electrons. Double and triple bonds can be created when two or three such types of pairs are shared. A Lewis structure is an illustration of a molecule or complex ion that depicts the disposition of valence electrons (inner shells are not drawn) around each atom. 1-4 depict Lewis structures of CH4, H2O, O2 and N2, the last two molecules consisting of a double and triple bond, correspondingly. These representations are completely equivalent to the valence structures (1′-4′) where each bonding pair of electrons is depicted by a line.
A molecule like H2O has nonbonding or lone-pair electrons localized on one atom rather than shared. The existence of these has significant consequences for both the shape of a molecule and its chemical properties.

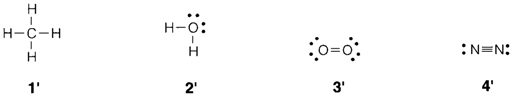
Simple complex ions like tetrahydroborate (BH-4 6) and ammonium (NH+4 5) can be drawn in a identical way; the valence structures displayed are necessarily similar to those for CH4 as the total number of valence electrons is similar in all examples. The isoelectronic principle suggesting that the molecules or ions having similar number of valence electrons should have identical valence structures, even though this idea has limitations.