Potentiostatic Coulometry:
Within potentiostatic coulometry, that is, coulometry at constant potential, the potential of the working electrode is maintained at a constant level, so that only analyte gets quantitatively oxidized or reduced there is no involvement of the less reactive species.
At this time the current is initially high but decreases exponentially with time and approaches zero at a time required to attain a reaction position that is quantitatively complete (~10-6 M).
Q might be evaluated through a current time integrator or graphical, Q = ∫ t0 idt as shown in Figure.
Area = Q = ∫ t0 idt
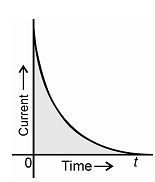
Figure: Current and time relationship in potentiostatic coulometry