Polarisation:
Let Consider again the condition described in Figure. If we additionally increase the Eapplied, a current becomes independent of Eapplied. This provide rise to limiting current as described in Figure. At this situation, the electrode is said to be fully polarized, since its potential could be changed widely without affecting the current. Polarisation could be conveniently divided within two kinds: concentration polarisation and kinetic (chemical) polarisation. It is an electrode phenomenon which might affect either or both of the electrodes in a cell.
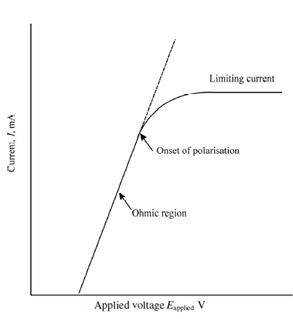
Figure: Current potential curve for electrolyte illustrating ohmic region (linear portion of the curve) and limiting current situation