Efficiency of electrodialytic procedure:
The efficiency of electrodialytic procedure is given in terms of Coulomb efficiency (η).
η = Number of equivalents of electrolytes displaced from dialysate to the brine/ Number of Faradays of electricity passed in the process
The unwanted transport processes, namely, coion transport, free electrolyte diffusion and water transport contribute to the reduce in Coulomb Efficiency.
The rate of counter ion transfer flux (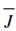 ) by membranes in electrodialysis procedure is given through
) by membranes in electrodialysis procedure is given through
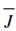 =
= 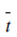 i /F
i /F
While, i is the current density (amperes per square centimetre field of membranes) and F is the Faraday constant.
Same, the electrical ion fluxes (J) in the solution is given through
J = t i / F
Eqs. 5 and 6 denotes that the counter ion fluxes which solution as well as in the membranes increase along with increase in current density.
The flux of ions computing from diffusion could be expressed in terms of Fick's first law.
[J]d = - D dc/ dx
At steady state, the joint electrical and diffusive flux by the membranes within the boundary layers equivalent the electrical flux by the membranes and the electrical flux by the membrane is the total flux.
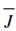 =
= 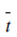 i/FZ = - D dc/dx + t i / FZ
i/FZ = - D dc/dx + t i / FZ
Integration of Eq. 11.55 yields a simple relationship among boundary layer thickness, δ, current density, i, interfacial concentration, Co, and the bulk concentration , Cb.
Co = Cb + (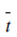 - t) iδ / DFZ
- t) iδ / DFZ